Carbonation
Carbonation occurs in concrete because the calcium bearing phases present are attacked by carbon dioxide of the air and converted to calcium carbonate.Cement paste contains 25-50 wt% calcium hydroxide (Ca(OH)2), which mean that the pH of the fresh cement paste is at least 12.5. The pH of a fully carbonated paste is about 7.
The concrete will carbonate if CO2 from air or from water enters the concrete according to:
Ca(OH)2 + CO2 → CaCO3 + H2O
When Ca(OH)2 is removed from the paste hydrated CSH will liberate CaO which will also carbonate. The rate at which this occurs is a function of concrete quality, in particular the cement content, the water/cement ratio and the compaction. It is generally accepted that the rate of the carbonation reaction is inversely proportional to the square root of the age of the structure.
The carbonation process requires the presence of water because CO2 dissolves in water forming H2CO3. If the concrete is too dry (RH <40%) CO2 cannot dissolve and no carbonation occurs. If on the other hand it is too wet (RH >90%) CO2 cannot enter the concrete and the concrete will not carbonate.
Rate of carbonation of concrete depends on:
- rate of diffusion of CO2 into concrete
- permeability of concrete by water
at RH of 50-70%, carbonation rate is the highest
low RH reduces reaction of Co2 with Ca(OH)2
high RH reduces rate of diffusion of CO2
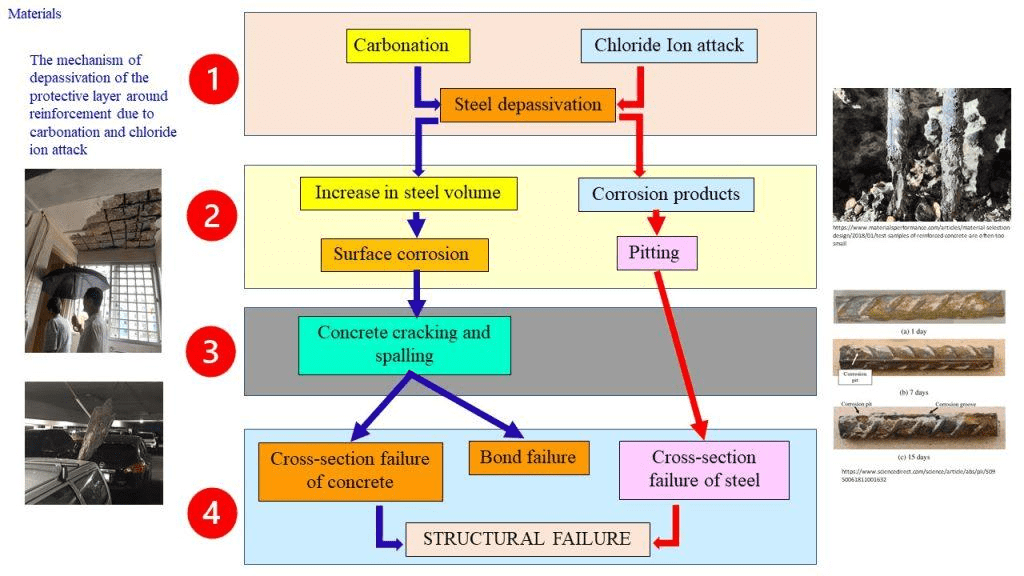
Normal carbonation results in a decrease of the porosity making the carbonated paste stronger. Carbonation is therefore an advantage in non-reinforced concrete. However, it is a disadvantage in reinforced concrete, as pH of carbonated concrete drops to about 7; a value below the passivation threshold of steel.
When pH is low, the metal irons in reinforcement, release at the anode pass into solution in the electrolyte. This results to form rust over the cathodic region. This transformation of metallic iron to rust is accompanied by an increase in volume. This volume increase is the principal cause of concrete cracking and spalling. Such carbonation also increases shrinkage of the concrete and then lead to cracking.
Recognition of Carbonation
Carbonation may be recognized in the field by the presence of a discolored zone in the surface of the concrete (Figure 1). The color may vary from light gray and difficult to recognize to strong orange and easy to recognize. Carbonation can be visualized by using phenolphthalein (Figure 2). In the optical microscope carbonation is recognized by the presence of calcite crystals and the absence of calcium hydroxide, ettringite and unhydrated cement grains (Figure 3). Porosity is unchanged or lower in the carbonated zone.


