Reinforcement Corrosion
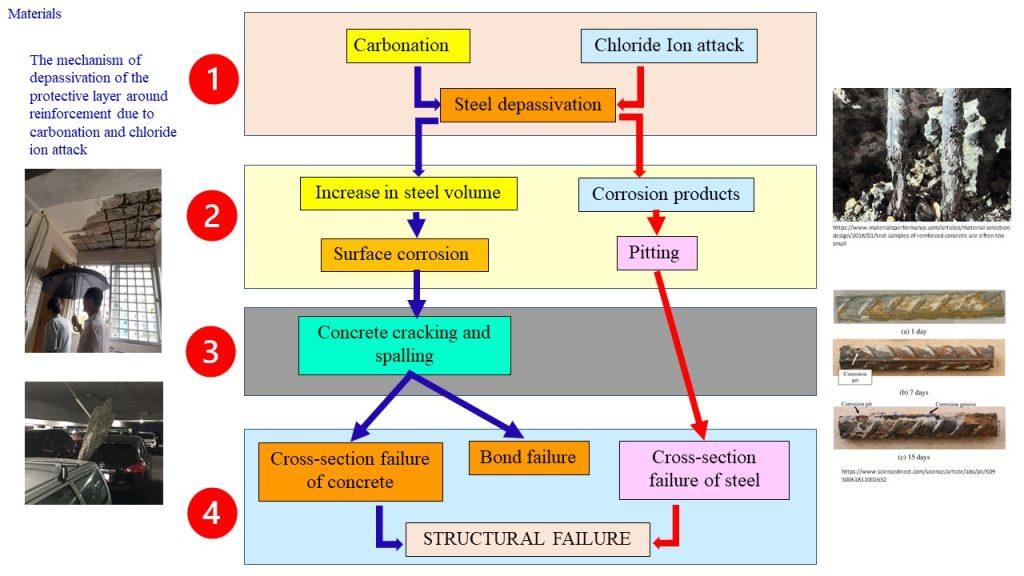
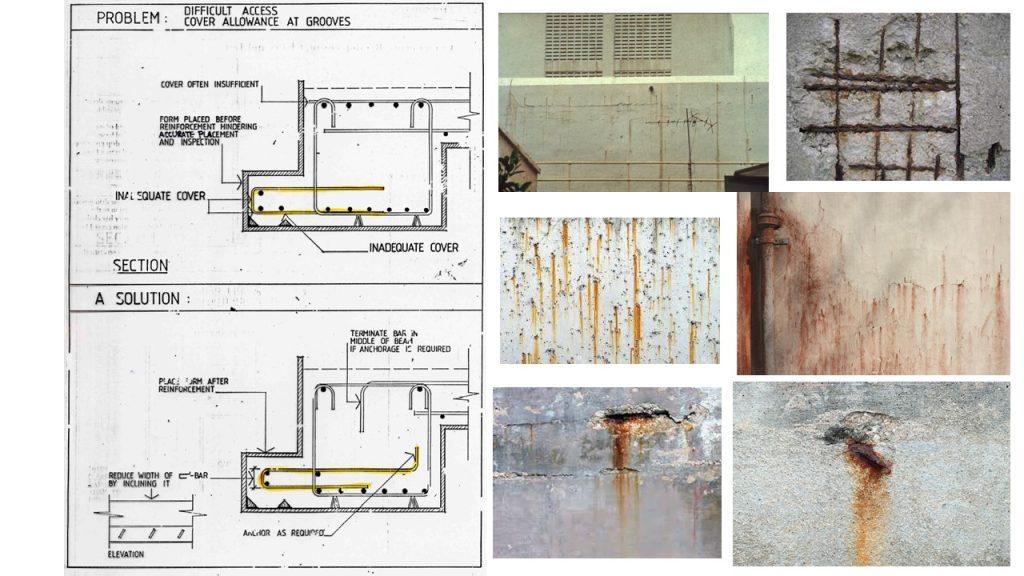
Corrosion is an electrochemical process, which can produce brownish colour deposits (rust) accompanied with increase in volume.
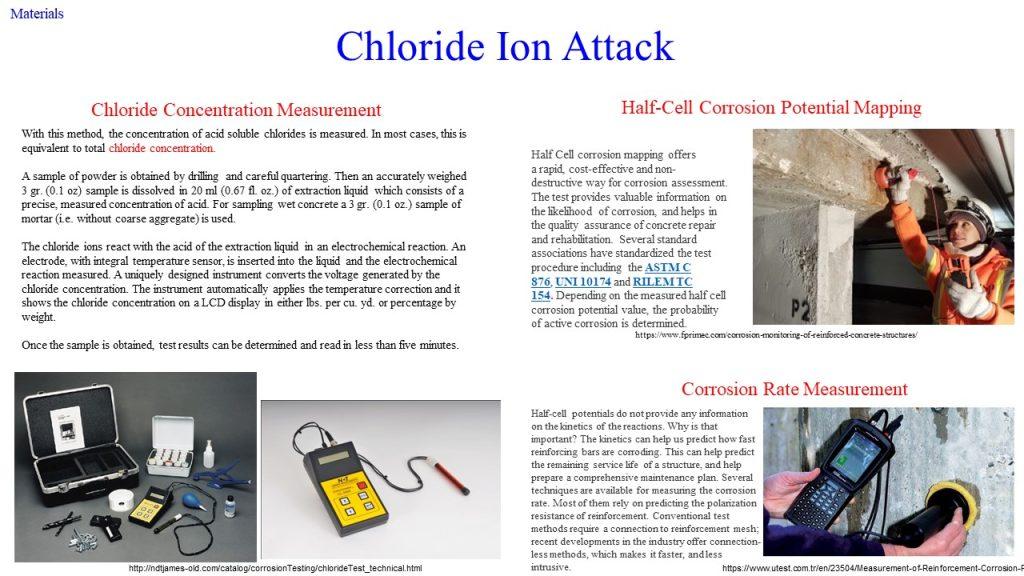
It represents a galvanic element – similar to a battery – producing an electric current, measurable as an electric field on the surface of the concrete. This potential field can be measured with an electrode known as half-cell. By making measurements over the whole surface, a distinction can be made between corroding and non-corroding locations. There are many scientific studies describing this method which has been known for more than 30 years.
As the rebar corrodes, rust forms and takes up more volume than the steel it replaces. This exerts expansive forces on the concrete, which ultimately leads to cracking, delamination and spalling.
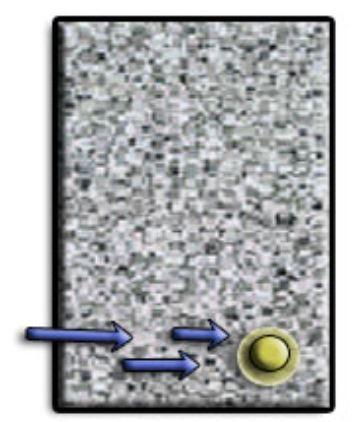
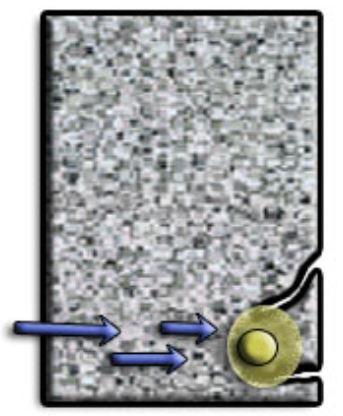
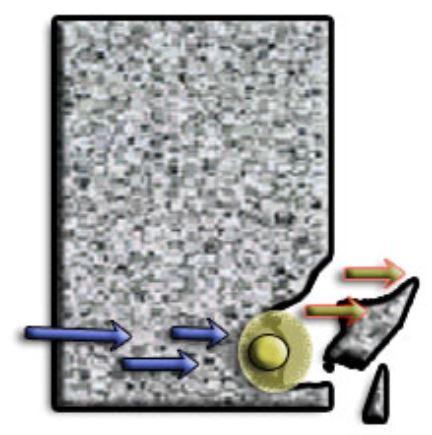
The 3 common reasons causing the reinforcement to corrode in RC are:
(1) Carbonation
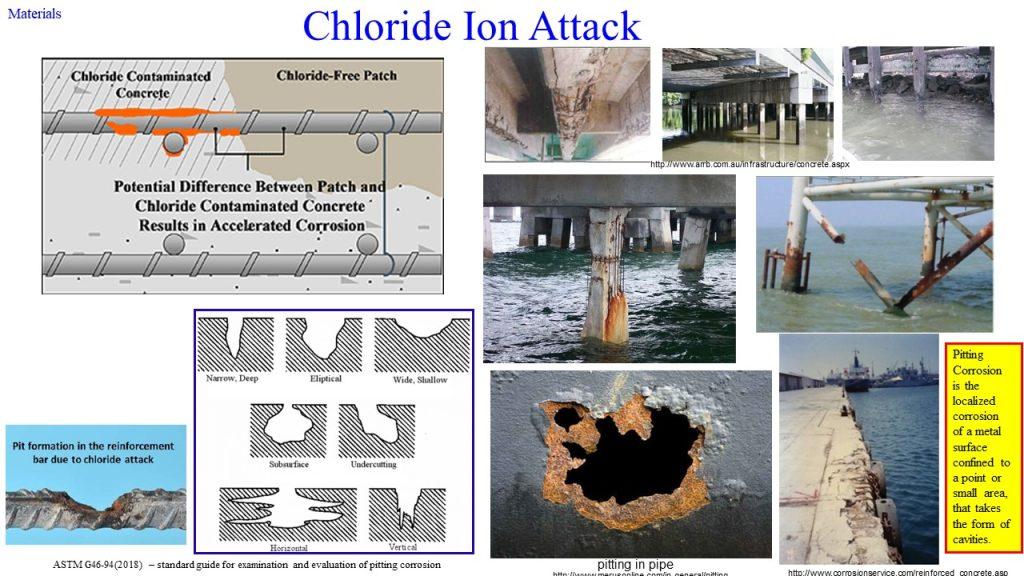
(2) Cl– ion penetration
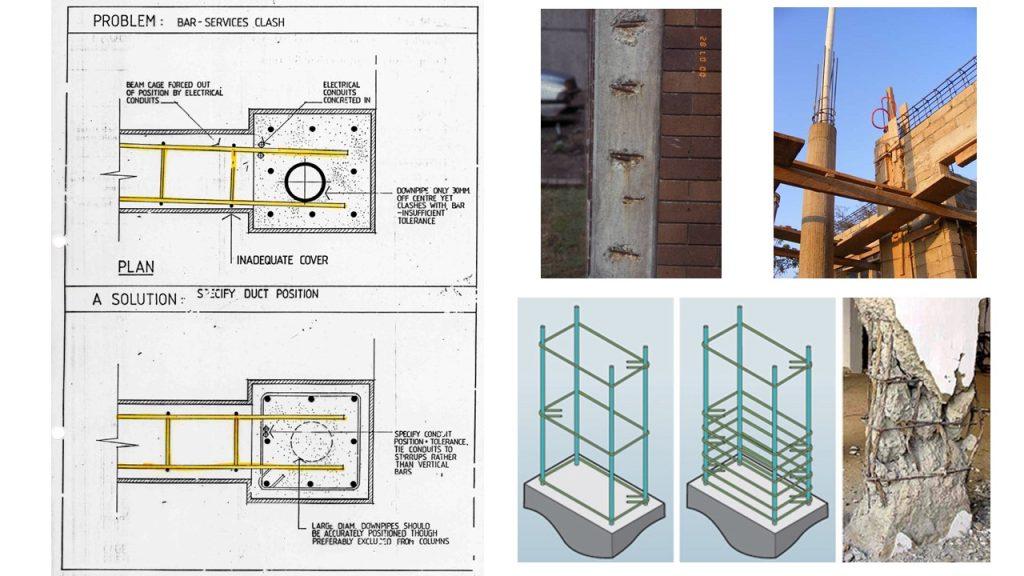
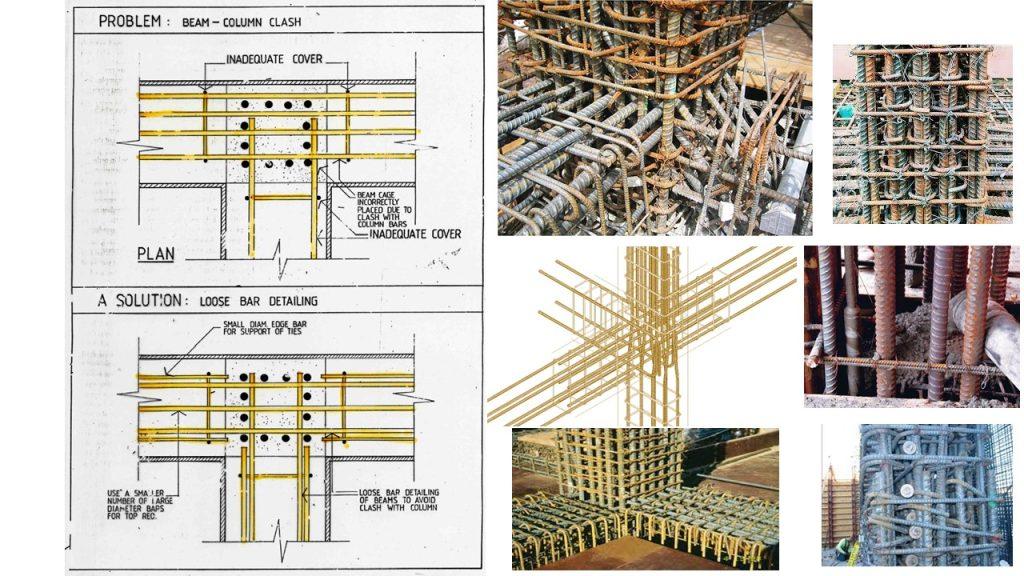
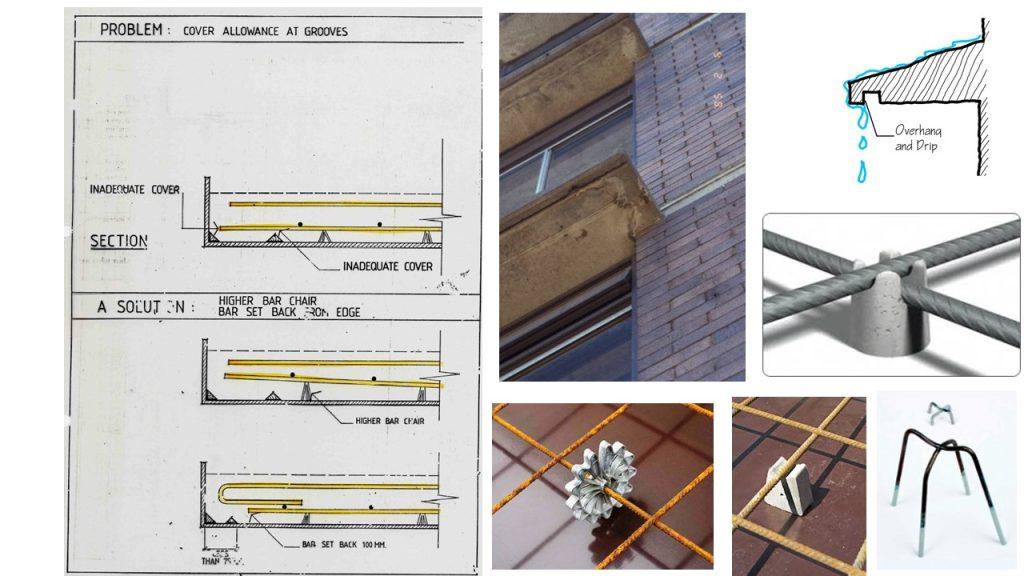
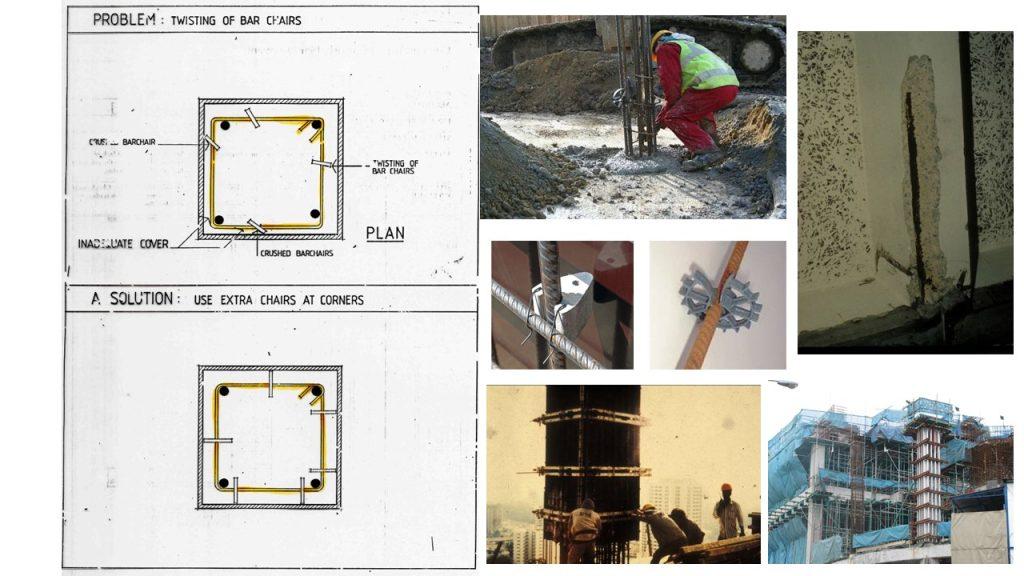
(3) Inadequate concrete cover (both quality and quantity)