Case 3
- Introduction
- Causes of Defects
- Good Practices
- Standards
- Maintenance and Diagnostics
- Remedial
- Similar Cases
- References
Cause of Defects
Sulphate attack on concrete involves a chemical process where sulphate ions break down the cement paste. This is caused by water-soluble sulphate salts, such as those containing alkali-earth metals (calcium, magnesium) and alkali metals (sodium, potassium), which react with the concrete. When sulphates penetrate concrete, they react with hydrated calcium aluminate and/or calcium hydroxide in the hardened cement paste, leading to the paste’s degradation. As sulphates dry, they form new expansive compounds called ettringite. These crystals take up space within the concrete, causing cracking and further deterioration. The expansion and needle-like shape of ettringite crystals create internal stresses, leading to cracking. Sulphate attack can also be physical, with water-soluble salts moving through the concrete and depositing on the surface, causing scaling and spalling. Sulphate ions can come from internal or external sources. Internal sulphate attack happens when sulphate ions are already present in the concrete mix, such as from sulphate-rich aggregates, excess gypsum in the cement, or sulphate-containing admixtures. Proper screening and material testing can usually prevent internal sulphate attacks. External sulphate attack is more common, typically resulting from high-sulphate soils and groundwater or from atmospheric and industrial pollution. Seawater is considered to have a moderate level of water-soluble sulphate ions.
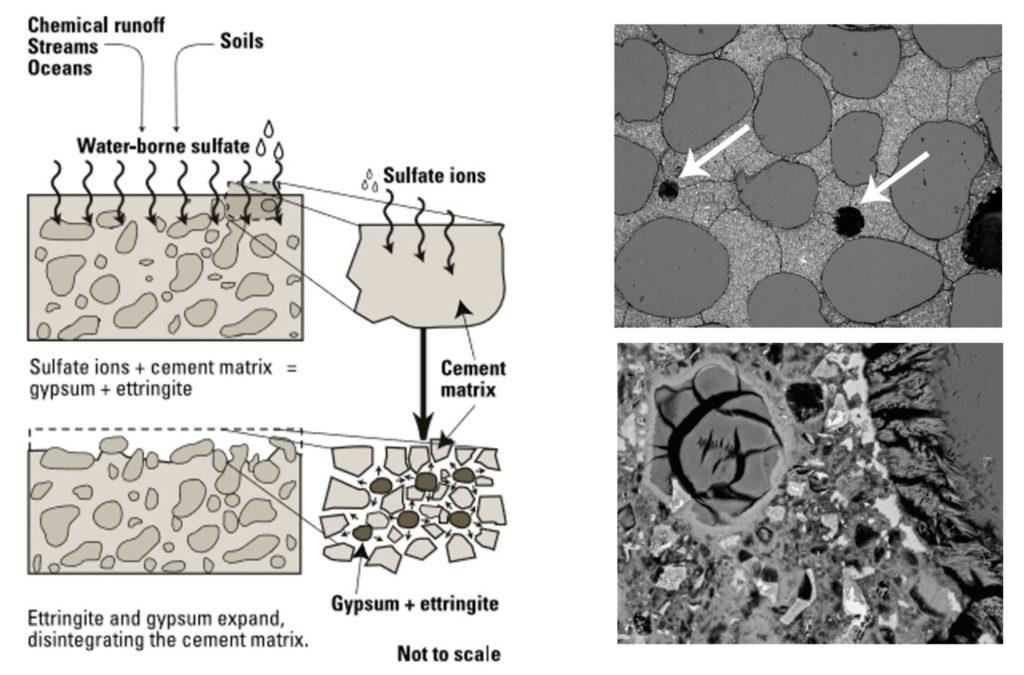
Sulphate attack generally leads to the formation of micro-cracks in concrete, resulting in surface scaling, disintegration, and eventually, significant deterioration. Indicators of sulphate attack include salt buildup on concrete surfaces, surface scaling, spalling with salt traces, de-bonding between aggregates and paste, and the concrete texture becoming toothpaste-like. These signs appear in the advanced stages of sulphate attack. This phenomenon is mostly observed in concrete elements that are buried or near the surface, such as piers, columns, slabs, foundations, and basements, where sulfate contamination is present in the surrounding soil or water.

Sulphate attack is caused by sulphates in solution. It results in an expansive chemical reaction between the sulphate and the concrete constituents. The sulphates penetrates the concrete and the reaction products first cause the concrete to gain strength as unoccupied pore space in concrete is being filled up. Disruption and final disintegration of the concrete soon follows.
Sulphate sources may be from groundwater which may rise up the concrete wall due to capillary action.
Common sulphates responsible for sulphate attacks:
- magnesium sulphate
- calcium sulphate
- sodium sulphate
- sulphuric acid
Consequences
The results of chemical attacks are:
- loss of strength of concrete
- loss of mass
- formation of ettringite
- increase in porosity and permeability in concrete
- reduction in durability
- worsening appearance, spalling of aggregates and other minor results.