Case 2
- Introduction
- Causes of Defects
- Good Practices
- Standards
- Maintenance and Diagnostics
- Remedial
- Similar Cases
- References

Introduction
Type of Building: Public
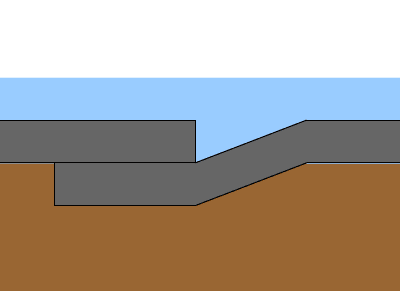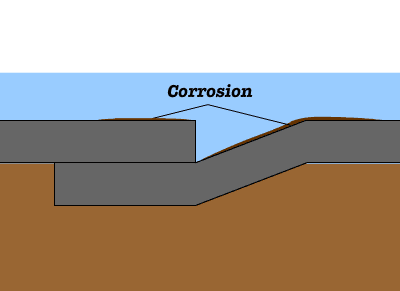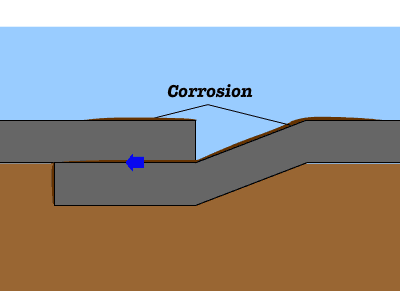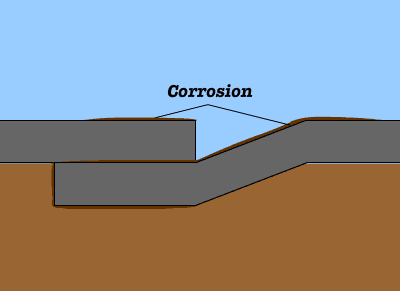
Cast iron waste pipe has been badly corroded and leakages were observed in this case. The use of cast iron in wet areas, is to serve the tub, lavatory, and water closet fixtures.
Cast iron is an iron-based material with a high percentage of carbon. Common problems encountered with cast-iron construction in wet area include rust. Rusting occurs rapidly when cast iron is exposed to moisture and air. Corrosion is an electrochemical process, in which following reactions are taking place to produce rust. Once a rust film forms, its porous surface acts as a reservoir for liquids, which in turn causes further corrosion. If this process is not arrested, it will continue until the iron is entirely consumed by corrosion.
The Home Improvement Programme (HIP) offers residents essential and optional improvements to improve the internal living environment and address some of the common maintenance issues in older flats. Essential improvements, which are fully funded by the Government for Singapore citizen households, are necessary for public health, safety or technical reasons. They include the replacement of cast iron soil/waste pipes, spalling concrete repair, and replacement of existing pipe sockets with new clothes-drying racks. Optional improvements include toilet upgrading as well as the replacement of entrance door/gate and refuse hopper, where residents need pay only for the items they have chosen at a highly subsidised rate.
As cast iron pipes can corrode over time, they are replaced with unplasticised polyvinyl chloride pipes under the HIP.
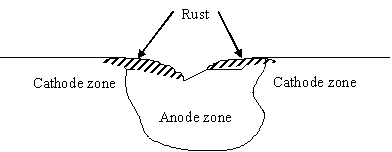
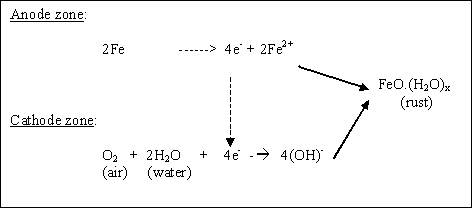
Cast iron is an iron-based material with a high percentage of carbon. Common problems encountered with cast-iron construction in wet area include rust. Rusting, occurs rapidly when cast iron is exposed to moisture and air. Corrosion is an electrochemical process , in which following reactions are taking place to produce rust.
Once a rust film forms, its porous surface acts as a reservoir for liquids, which in turn causes further corrosion. If this process is not arrested, it will continue until the iron is entirely consumed by corrosion.
