Case 1
- Introduction
- Causes of Defects
- Good Practices
- Standards
- Maintenance and Diagnostics
- Remedial
- Similar Cases
- References
Cause of Defects
The corrosion of brickwork by sulphates may be accelerated if the sulphates migrate in aqueous solution and the brickwall is saturated and percolate down into the mortar beds.
Possible sources of water:
a) Rainwater
The primary source of moisture for the occurrence of efflorescence is rainwater (acid rain), which penetrates or comes in contact with masonry. Water film adheres to the external surfaces of wall and migrates via capillarity through the brickwork.
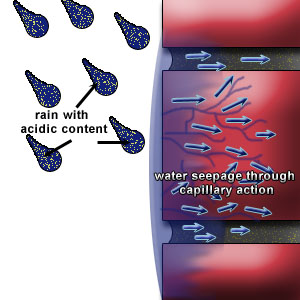
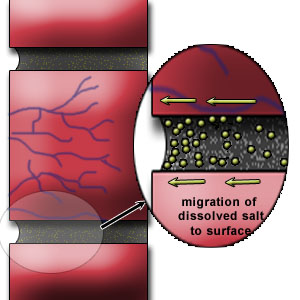
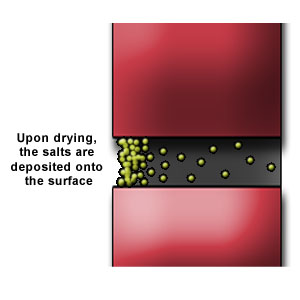
The presence of this free water in mortar joints could lead to the dissolution of alkaline salts available. Upon drying lime from the mortar is brought to the surface. (Figure 1a, b & c)
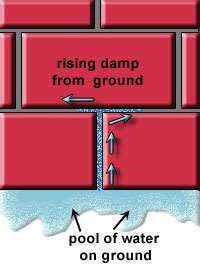
From the picture, the lime bloom occurs not far from the ground DPC level. Another possible contributing factor of moisture is ground water carried up a wall (Figure 2)
c) Condensation
In addition to rain water and ground water, water may accumulate within the wall as a result of condensation of water vapor.
Condensation is usually due to moisture originating inside buildings. Cold air from internal air con unit may condense on the interior surface.This gain in moisture content increases the vapor pressure of the inside air substantially above that existing outdoors. This increased pressure tends to drive the vapor outwardly from the building interior through any vapor-porous materials that may comprise the enclosing surfaces.
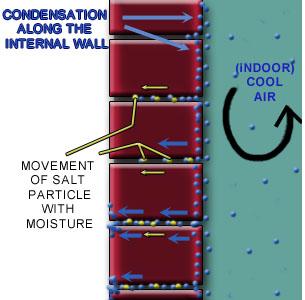
When vapor passes through porous and homogeneous materials, mortar in this case, which may be warm on one side and cold on the other, encourages condensation. This condensed moisture can contribute to lime leaching on the mortar joints (Figure 3).
These may cause mortars that have been incorrectly specified or batched and have a low cement content to deteriorate under sulphate attack. Sulphates from the ground or other sources may be equally destructive.
Sources of sulphates can be from:
- Sulphuric acid solution developed from atmospheric conditions, especially in industrial areas dissolves the brick at a rate approximately ten times higher than it will be dissolved by pure water.
- However, the resulting salts are more damaging to the appearance of the building than to its actual strength, leaving staining and efflorescence on the masonry structure as shown.
- Moisture in a wall from rising damp, rainwater, and condensation may carry acids which attack the bricks and mortar. The water may also carry soluble salts that subeffloresce, or crystallize, in the pores of a brick, causing spalling of the brick surface.
- With repeated wetting and temperature cycling, crystallize salts will produce an expansive force sufficient to disintegrate the unit. Acids in the water may also adversely affect the anchors.
Consequences
This process gives a net expansion and causes both local disruption of the mortar bed and stresses in the brickwork and causes severe expansion .
Reaction of such liquids with various elements in the masonry may result in both formation of hardened surface crusts and dissolution of acid-soluble cementing binders that hold the masonry together. The formation of dense surface crusts and the washing away of acid-soluble binders, if left unchecked and removed, are progressive deterioration processes.