Case 2 – Chloride Ion Attack
Navigation
- Introduction
- Causes of Defects
- Good Practices
- Standards
- Maintenance and Diagnostics
- Remedial
- Similar Cases
- References
Cause of Defects

In marine locations:
Accumulation of salt spray on reinforcement and formwork ahead of concreting, and be incorporated into concrete during placement.
In coastal and near coastal areas:
Deposition of salt spray onto hardened concrete surface and the salt can permeate into the concrete.
For architectural purposes:
Surface of concrete may been etched with hydrochloric acid to expose the aggregate or cleaned to receive architectural finishes.
Mechanism:
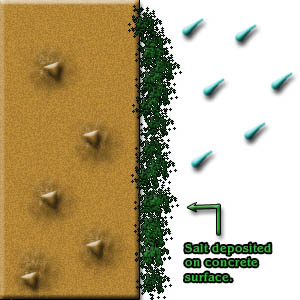
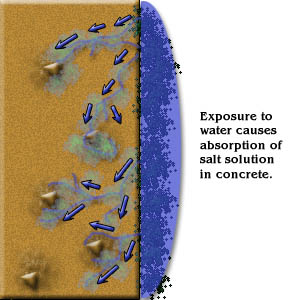
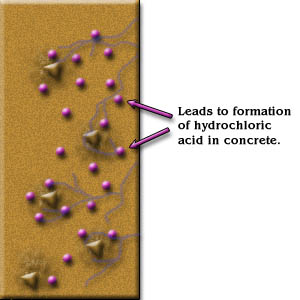
- Soluble chlorides to combine with water in the “pores” of concrete to form hydrochloric acid
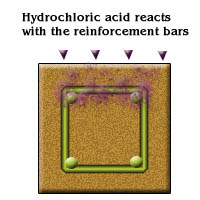
- Hydrochloric acid reacts with reinforcement to form ferrous chlorides
- This ferrous chlorides subsequently breaks down into form of ferrous hydroxide and hydrochloric acid for further cyclic reaction
Consequences
The results of chloride attacks are:
- reduction of passivity of the concrete conditions, promotion of corrosion of steel reinforcement in concrete
- resultant rust formation occupies a volume that is several times that of the reinforcement bar, causing cracking and eventually spalling of concrete.
Risk of reinforcement corrosion as a result of chloride contamination depends on:
- concentration of chloride
- alkalinity of concrete
- type of cement
- whether chloride was present at time of mixing
- whether chloride has penetrated the hardened concrete