Case 1 – Alkali-Silica Reaction
- Introduction
- Causes of Defects
- Good Practices
- Standards
- Maintenance and Diagnostics
- Remedial
- Similar Cases
- References
Cause of Defects
A mechanism of deterioration that results from an interaction between alkaline pore fluids in the concrete and certain types of aggregates of high silica materials [4].
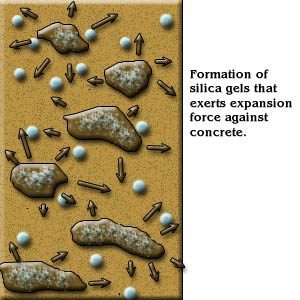
The alkali reaction with siliceous aggregate forms a silica gel, which imbibes water, producing a volume expansion that disrupts the concrete.
Conditions for ASR to take place [3]:
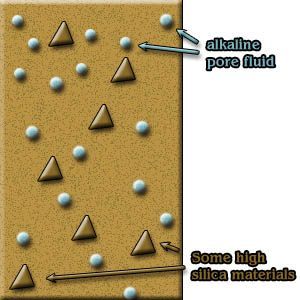
- A certain amount of alkali-reactive aggregate must be present: sodium and potassium alkalis or reactive silica.
- The cement paste pores must contain solutions with sufficient alkali hydroxide. Sources may include sea salt spray and the cement used.
- The concrete to be exposed to continuous moist or wet conditions.
Consequences
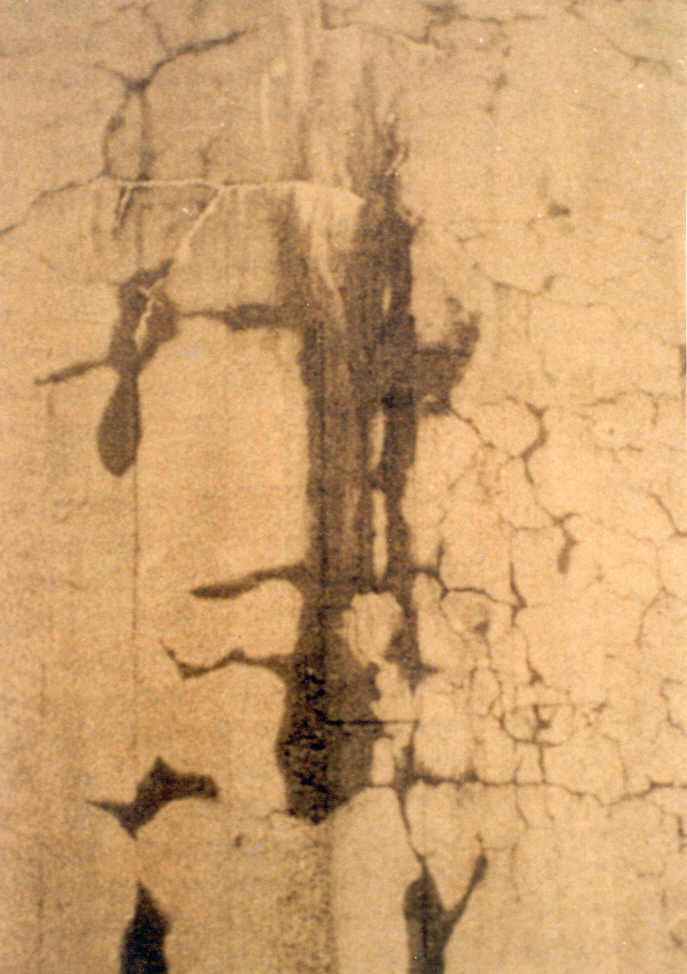
The reaction can induce cracking and expansion in concrete, particularly in high quality concrete members exposed to external moisture. It creates a calcium alkali-silica gel, which is hygroscopic so that in the presence of water, it will cause considerable volume expansion (Figure 1). This expansion weakens the concrete, causes dislocation, distortion and misalignment of structural concrete [1].
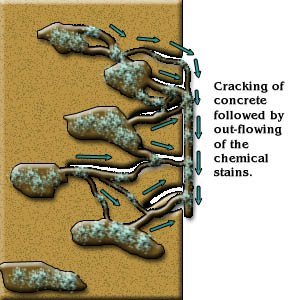
Cracking initiated due to the expansive reaction of silica products may perpetuate and permits further ingress of water and dissolved salts, leading to consequent corrosion of the reinforcement.

In the advanced stages of the reaction and cracking has become extensive, the compressive strength of concrete will reduce drastically. In elevated structures, there may even be danger of spalling of concrete (Figure 2).