TiO2 Coating
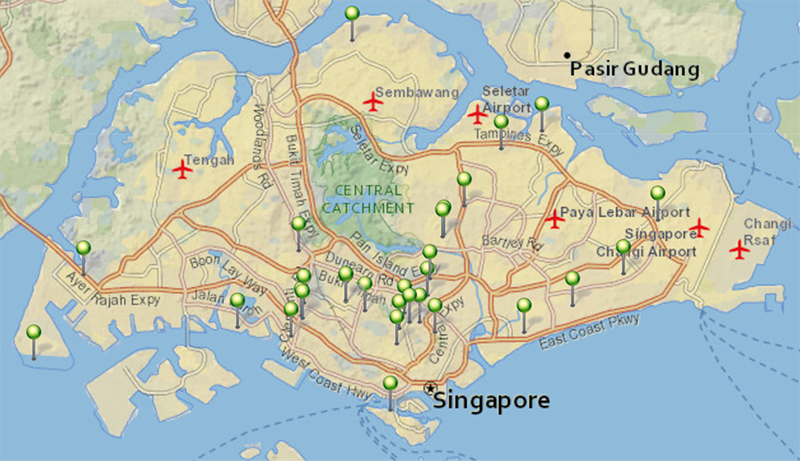
Location of Façade Coatings in Singapore
Introduction
Dirt collection and accumulation on building façade have been a persistent problem for facility managers and building owners because it affects the aesthetics of the building exterior outlook. The cleaning of such building surface is generally done by using detergents accompanied with scrubbing or high-pressure water jet which led to high maintenance cost. Thus, the self-cleaning ability of the Nanostructured Titanium Dioxide (TiO2) coating has gained considerable attention in the industry concerning this common problem1. TiO2 nanoparticles are primarily in the 1 to 100 nanometres size range and are transparent and not inert3. TiO2 coating is used as an anti-staining coating on building facades and is developed using nanotechnology5 to improve facade25 maintainability since the organic compounds that adhere on facades are easily washed off due to the bond broken with dirt particles24.
Main commercial applications of titanium dioxide with sunlight
| Commercial field | Function | Use |
| Glass | Self-cleaning, antibacterial, and antifogging | Windows, mirrors, and vehicle glasses |
| Vehicles | Self-cleaning, antibacterial, antifogging, and avoiding dewdrops | Coating and painting of vehicles, and side-view mirror |
| Textiles | Self-cleaning and antibacterial | Tent materials, storage structures, and stations and domes |
| Food and flowers | Antibacterial and anti-aging | Fruits and flowers preservation |
| Buildings, hospitals, and concrete | Self-cleaning, antibacterial, and antifogging | Kitchen, bathrooms, exterior tiles and coating, and walls and roofs |
| Road materials | Self-cleaning and antifogging | Road pavements and mirrors, sidewalks, and lighting and signs |
| Water treatment | Decontamination, antibacterial, and mineralization | Small industrial and agricultural wastewater treatment plants |
| Industrial chemistry | Selective oxidation | Fine chemicals solar productions |
(Source: Chen & Poon, 2009)
Properties of TiO2
Self-cleaning mechanism
When TiO2 is exposed to Ultra Violet (UV) rays (320-400nm), it produces hydroxyl radicals (OH) and superoxide ions (O2-) which are highly oxidative compounds that can disintegrate dirt and organic substances. Photocatalysis of TiO2 also reduces the contact angle between water droplets and a given surface, to form a super-hydrophilic surface, which increases the self-cleaning ability20. This is so as water forms thin sheets on top of the substrates which washes off broken-down organic matter in one movement6,9. Photocatalytic process for hydrophilic self-cleaning glass is depicted in Figure 1. Grease, dirt and other staining particles can be removed in the presence of water. Super-hydrophilicity, combined with the strong photocatalytic oxidizing properties, makes facades coated with photocatalytic coating perform self-cleaning functions24.
When illuminated by ultra-violet light, external surfaces to which nanostructured TiO2 coatings are applied to would exhibit super-hydrophilic behaviour, attracting water to form a uniform sheet over the surface at a contact angle of 70 (exterior) and 250 (interior) and preventing adhesion of surface particles. Decomposed compounds and inorganic molecules will be swept away with water. The surface would then dry off quickly without leaving any signs of streaks4. Arbab et al. (2005)2 report that this photo-induced hydrophilicity is probably the result of reaction between photo-generated holes with bridging oxygen species at TiO2 surface to form sites. This causes dissociative adsorption of water at the defect sites and eventually an increase in the hydroxyl groups at the coating surface21.

Figure 1 (A) Dirt adheres onto the hydrophilic self-cleaning glass (left) (B) Photocatalytic activity is activated by UV light and organic dirt is decomposed (middle) (C) Upon contact with rainwater, it washes off the organic dirt
(Source: Pilkington, 2012)
As shown in the Figure 2, the self-cleaning mechanism of TiO2 is derived from both oxidative (photocatalytic) and hydrophilic nature. When exposed to sunlight, the strong oxidation power will decompose organic substances, whilst the hydrophilic nature of the coatings would naturally allow water or rainfall to easily wash away dust and dirt particles. Odours in the air would also be eliminated. Together, they form a self-cleaning mechanism that prevents adhesion and built-up of both inorganic and organic compounds, thus retaining a clean surface21. In addition, as the number of photons available in the coatings limits photocatalysis, its hydrophilic property allows the surface to be maintained at a clean state regardless of whether there is ample number of photons to break down contaminants adhering to the surface16.
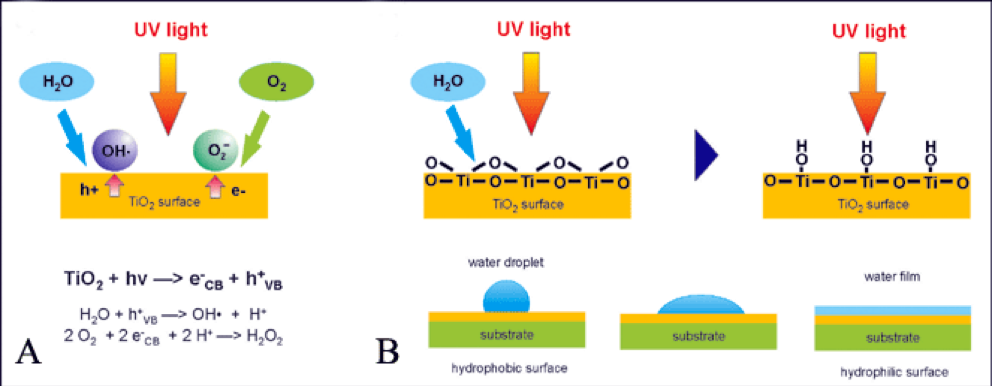
Figure 2 Photocatalytic process and hydrophilic action of nanostructured TiO2 coating
(Source: Research Centre for Nanosurface Engineering, 2006)
Air Pollution Reduction
In 2006, the PICADA (Photo-catalytic innovative coverings applications for dee-pollution assessment) project which aims to contribute to the development of TiO2 based coatings for self-cleaning and air pollution reduction coatings reports that their experiment result showed a reduction in NOx emission between 40% to 80%15. Moreover, Pacheco et al. (2011)20 report that in a study by Maggos et. al. in 2008, the emission reduction is found to be 36.7% and 42%. When exposed to light, TiO2 (see Figure 3), a semiconductor, undergoes an activation process through the absorption of a photon, which energy exceeds that of the band gap. This causes the promotion of an electron from the valence band to the conduction band, in turn generating a hole in the valence band. This hole oxidizes the adsorbed donor molecules (for example pollutants and volatile organic compounds) while the conduction band electrons reduce the electron acceptor molecules. The final products of this chemical reaction are carbon dioxide and water29.
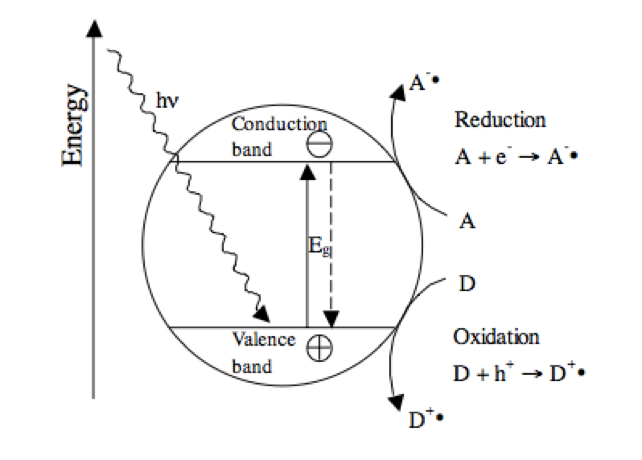
Figure 3 Operation of a photochemical excitedTiO2 particle
(Source: Benedix, Dehn, Quaas, & Orgass, 2000)
Being an ultra-violet light attenuator and not as effective under visible light, the adoption is better taken on outdoor surfaces, breaking down airborne pollutants in the environment. However, where indoor ultra-violet lighting is available, TiO2 coatings can also be adopted on indoor surfaces to neutralize indoor contaminants and improve indoor environmental quality21. For instance, the Jubilee Church (see Figure 4) which is designed by Richard Meier features large white curved TiO2 -coated external walls. The building is found to significantly reduce petrochemical smog, originating from the major roads in the vicinity28.

Figure 4 Jubilee Church by Richard Meier
(Source: http://www.doobybrain.com/2007/09/19/jubilee-church-by-richard-meier/)
Enhance indoor air quality
A hospital case study in Singapore has implemented TiO2 and UVC emitters in their pre-cooled AHU system such that they are able to enhance indoor air quality within the building26. This is an innovative application, rather than implementing on the interior walls because it cleans the air before the air travels to the various zones in the building, which is more efficient. Therefore, implementing TiO2 in the AHU system is a good move as it cleans the air thoroughly before allowing the clean air to move towards the various zones in the hospital. The TiO2 layer will also be efficient because there are UVC emitters installed in the system that provides sufficient UV radiation for the efficient performance of TiO2’s photocatalytic function26.
Bactericidal Properties
Hydroxyl radicals are primarily responsible for the bactericidal capacity of TiO2 where hydroxyl radicals possess a destruction capacity of Escherichia Coli bacteria, which makes it 1000-10,000 times more effective than chemical disinfection products7. Another hospital case study has implemented TiO2 in the interior of their building as an experiment to test whether or not the contamination of surfaces will be lowered comparing the surfaces that were TiO2 coated and were not TiO2 coated over the course of 2 years8. It was tested that TiO2 was effective over this period and that the levels of contamination were reduced. Specific locations in the hospital were selected to test the effectiveness of TiO2, and findings have shown that in areas treated with TiO2, there is lesser frequency of having bacteria on the location’s surface. Therefore, TiO2 is seen to be efficient in hospitals, where hygiene is extremely important especially zones such as the Intensive Care Unit26.
Types of Application for TiO2
Exterior Applications
One reason that TiO2 is used outdoors is due to its effective performance of self-cleansing. When TiO2 is applied to the glass of curtain walls, it will be exposed to sunlight on the exterior of buildings. When sufficient amount of sunlight activates the photocatalytic process, the stains (dirt and dust from the exterior of buildings) can be broken down and is easily washed away when the rain comes. This is because the water soaks between the broken down compounds and the highly hydrophilic TiO2 surface16. This is not limited to only glass; other building materials can be used as well such as exterior tiles, aluminium walls and PVC fabric26. Another reason that TiO2 is used outdoors is the anti-fogging function. The difference in temperature on the outside and inside of building shows a great contrast, and when the heat from the exterior of buildings come into contact with the cold window or curtain wall surface, it will lead to fogging or condensation. On a highly hydrophilic surface, no water droplets will form on the surface; instead, a uniform thin film of water is formed on the surface that will prevent fogging or condensation16.
Interior Applications
One reason that TiO2 is applied indoors is due to the function of improving indoor air quality. This is done through reducing the organic chemicals that are present in the indoor space due to daily regular activities. These organic chemicals come from detergents, disinfectants, paint, perfumes, deodorants and cosmetics; these include nitrogen oxides, Volatile Organic Compounds (VOCs) and particulates6. This is beneficial for the building occupants; such that they are not subjected to side effects should they be exposed to these organic chemicals over a long period. Also, active photocatalytic surfaces can help in minimizing the proliferation of mould, bacteria and fungi in the building, which are present due to moisture in wet areas of the building. TiO2 can be applied in the filters of air cleaners, which will then kill bacteria that come into contact with it10. Photocatalyst equipped air cleaners can also kill the bacteria in the indoor air11, which is beneficial for buildings that prioritize the health of its occupants, such as hospitals, eldercare and educational institutions26. Another reason that TiO2 is applied indoors is due to its effectiveness in maintaining a clean environment within the building. As mentioned earlier, when TiO2 is applied on the interior surroundings of the building, the indoor air quality will be improved and bacteria present in the space will be destroyed when it comes into contact with the TiO2 surface14. Therefore, this is the ideal environment that a user can experience in the building; good indoor air quality as well as an environment that is almost free from bacteria and germs26.
Application onto different substrates and building components
Different type of substrates affects the efficiency of photocatalytic properties; hence it is paramount to understand the characteristics of each substrate before application of photocatalytic coating13. Many studies have shown the wide application of TiO2 coating being applied onto different substrates; concrete19, limestone22, heritage building’s stone surfaces19,29, tiles and glass24. For organic materials, it is unable to withstand the high temperature that is required to anchor the photocatalyst layer during manufacturing. It was observed that organic building materials tend to be decomposed by photocatalytic reactions, resulting in a decrease of photocatalytic activity18 and also damage to the structure and strength of the organic building materials6. An intermediate layer should be applied between organic materials and photo catalyst to prevent such situation from occurring resulting in an increase of the manufacturing cost6. It was proven that application of TiO2 coating applied on concrete at “Dives in Miseriocordia” church showed high resistance and durability towards environmental factors. With self-cleaning properties, it ensures a long-lasting white surface, which was evident in the constant colour of the façade throughout five years at four different positions (North/South/East/West)9. Photocatalytic concrete has the big advantage of maintaining its clear colour for a very long time, even if the corresponding building is situated in a very crowded and polluted town. For glass, photo-catalytic activity is stable18. Upon coating of TiO2 film on the glass, the primary condition is that the film should be highly active and stable27. For in-factory installation of photocatalytic activity of TiO2 film, it highly dependent on temperature, flow rate and partial pressure during manufacturing process17. It was proven that for on-site application, glass exhibits satisfactory self-cleaning effect, due to their smooth composition18.
There is an exceptionally wide range of application for nanostructured TiO2 coatings. Figure 5 shows some of the various building components nanostructured TiO2 coatings can be applied to21.
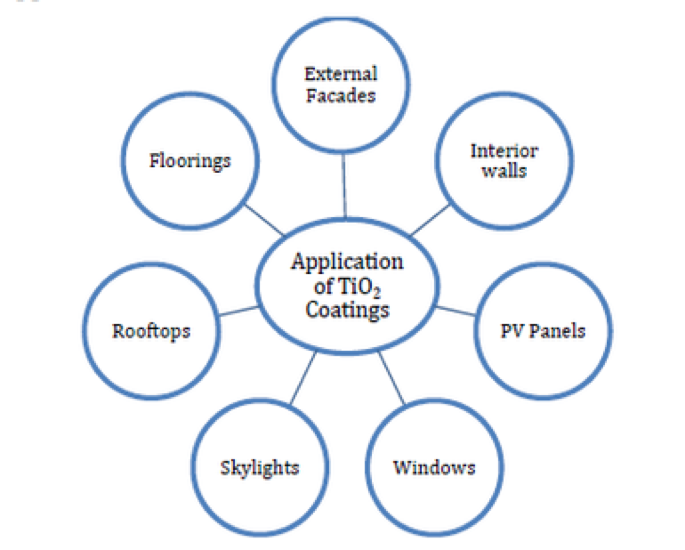
Figure 5 Examples of some building componentsTiO2 coatings can be applied to
(Source: Poh, 2011)